The principle method of Dumas in protein analysis and protein Nito
On this, with the breakthrough of technology developed, the principle of the Dumas method becoming increasingly popular all over the world in many fields: feed, food, raw materials, the environment, industrial production ...
The strict regulations and standards in quality management of nutrition in food, feed is being applied in Vietnam in particular and the world in General. The exact protein analysis enabling the product to be accepted in many international markets than, protein and economic interests that they bring.
The Velp Scientifica and Leca has given many lines by protein analysis machine Dumas principle. This method is superior to traditional Kjeldahl method. Principle of Dumas combustion method, also called the or element analysis used to detect total nitrogen and protein content.
The principle method of Dumas determine the nitrogen was developed in 1831 earlier, the principle of the Kjeldahl (1883) , more convenient in many characteristics such as: analysis of speed, environmental safety and user, high precision, performance and cost analysis of lower.
This time, application and development of the Engineering Sciences also are poorly so the application method of principle of dumas nearly done, so are many more application Kjeldahl (the method does not need many of the Advanced cell technology). But today, Dumas has gradually replaced the entire Kjeldahl method with multiple disadvantages: analysis of time too long, recovery of lower nito, low detection limit, toxic gases flying out human health effects, the high cost ...
The results obtained in determining gas nitrocarburizing Dumas Kjeldahl method is often higher a bit, because the compounds and compounds not yet nitrogen (for example: nitrite, nitrate) also to be discovered. Kjeldahl method in these compounds the ammonium ion NH4 + is converted into not should not detect. on the other hand there are many Kjeldahl variation affects the end result: error of titration, error break model, wrong number of distillation, chemicals ... because when breaking protein samples, pp Kjeldahl for much additional work: Concentrated H2SO4, K2so4, CuSO4..
But the speed of the recovery of the two methods is relatively the same (greater than 99.5%), While the limit discovered Dumas again outperformed (0.003 mgN) Kjeldahl only with bigger 0.1 mg N.
The principle of operation of the Dumas method
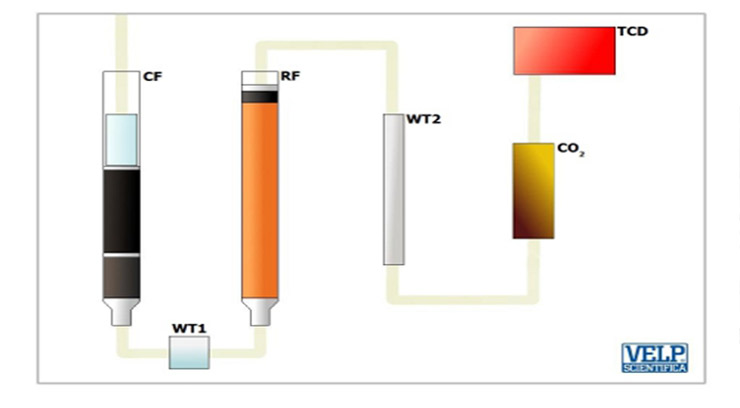
Define nito according to the principle of the method the same demanding Dumas for templates, combustion at high temperatures where the burning sample about 1000 C the presence of pure Oxygen gas. This process creates gas: H2O, CO2, NxOy ... This gas mixture is going through physical traps to remove H2O and gas left is NxOy be reduced by combustion chamber containing copper at temperatures 650 degrees C. The NxOy will turn into elemental nitrogen gas. N the same excess Oxygen Gas element, CO2 will be going through the Ministry of absorption to eliminate residual CO2, water. Nitrogen gas will be measured by the sensor Measuring electric conductivity-TCD.
This is an advanced automated techniques are likely quick measurement of total nitrogen concentrations in food samples. The type of samples measure: solids, Loose is the package on the foil in (maximum volume 1 gram).
03 steps of the Dumas method:
- Burn: The template is the correct weight and purification to remove nito gas in the air. Are burned at high temperatures, with the participation of pure Oxygen in 1000 degrees C. This will create the gas as: Carbon dioxide, water, nito oxide.
Model + O2 à CO2 + H2O + O2 + NxOy + Other oxides
- Remove and reduction of Oxygen: Fire Anima products go through the Chamber containing the same hot CU 650 C converts the molecule into Nito Oxides N2. The remaining gas is absorbed out through the Ministry of absorption.
CO2 + H20 + NxOy + O2 + Cu → CO2 + H20 + N2 → N2
- Measure: the measuring signal from TCD sensors according to the principle of electrical conductivity.
Protein Analyzer Dumas NDA 701 Velp using EDTA (9.57% Analysis of protein-protein using H2SO4) the standard way to do reference, Thanks to its high oxygen content should meet the full needs oxygen to the combustion chamber. However, You can use Aspatic Acid, acetanilide, Ure, Atropine and other chemicals to create a standard way to reference.
Using standard chemical depends on nitrogen content of user's analysis template. A good standard way your order minimum requirements 6 the benchmark in different levels. Sample sizes as small as the sample evenly exactly. So tips when using Dumas , the sample preparation process must be as carefully to avoid the big error.
Currently, both the method of Kjeldahl and Dumas have been recognized by the international standards: AOAC,EPA, DIN, ISO, AACC, ASBC, AOCS, OIV…
























Readers comments (0)